rfid chip mri safety It concludes: “According to the ASTM standard, a device is considered as MR-safe if it causes no known hazards to patients in all MR environments. Since the RFID tag contains conducting materials, RFID may only be MR-conditional, meaning safe under certain conditions for MR imaging during the scan. Significant increasing of temperature and .
We'd like to start by clarifying that you won't see the option to enable NFC .
0 · verichip microtransponder mri
1 · rfid mri safety
2 · mri safety topics
3 · mri safety articles
4 · mri safety
5 · is rfid safe for mri
Source code SDK software for NTAG® 213, NTAG® 215, NTAG® 216, and MIFARE® Ultralight tags. NTAG & Ultralight software with SDK for working with all types of NTAG2xx tags (NTAG213, NTAG215 and NTAG216) and MIFARE Ultralight.
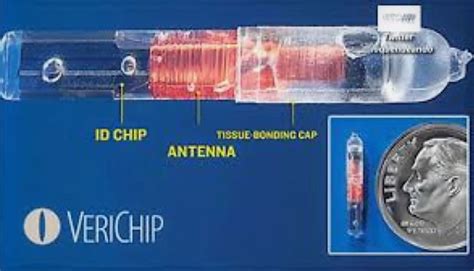
verichip microtransponder mri
contactless card oyster receipt
The VeriChip Microtransponder is a miniaturized, implantable radio frequency identification device (RFID). With regard to MRI procedures, the labeling for this device states: Patients with the VeriC.This is a tiny radio-frequency identification (RFID) tag that passively responds to an external hand-held transmitter/receiver probe. The device creates a mild (1 cm) artifact on MRI.The VeriChip Microtransponder is a miniaturized, implantable radio frequency identification device (RFID). With regard to MRI procedures, the labeling for this device states: Patients with the VeriC.This is a tiny radio-frequency identification (RFID) tag that passively responds to an external hand-held transmitter/receiver probe. The device creates a mild (1 cm) artifact on MRI.
The aim of this systematic review is to synthesise published information on MRI burns and safety-related guidance to develop comprehensive safety recommendations for best MRI practice to prevent burns.
It concludes: “According to the ASTM standard, a device is considered as MR-safe if it causes no known hazards to patients in all MR environments. Since the RFID tag contains conducting materials, RFID may only be MR-conditional, meaning safe under certain conditions for MR imaging during the scan. Significant increasing of temperature and . MRI safety. radiofrequency identification. RFID. R adio-frequency identification (RFID) technology has been in use for over 50 years. The technology involves a microchip attached to an antenna, which responds to an incoming signal from a reader by sending an outgoing signal. Although some implant devices with RFID tags may safely undergo MRI on the basis of formal safety testing, peer-reviewed literature on RFID chip safety and impact on image quality is scant, and MRI should be performed with caution [2]. The radio frequency identification (RFID) technology with tags (transponders) in wristbands and medical equipment may close this gap and increase patient safety. An RFID tag is a microchip attached to an antenna and usually fashioned in such a .
A recent development has been the labeling of the implants with a radio-frequency device micro responder chip (RFID). We examined a patient with silicone implants containing RFID chips with magnetic resonance imaging and were surprised by .
Patients wearing RFID wristbands are safe in 1.5 T and 3 T MR scanners using normal operation mode for RF-field. The findings are specific to the RFID tags that underwent testing. MRI safety and compatibility testing on the VeriChip™ radio frequency identification device was completed. The results were compared to several other biomedical implants to conclude if the device would be MRI safe and/or compatible weighing benefits and risks.The VeriChip Microtransponder is a miniaturized, implantable radio frequency identification device (RFID). With regard to MRI procedures, the labeling for this device states: Patients with the VeriC.
rfid mri safety
This is a tiny radio-frequency identification (RFID) tag that passively responds to an external hand-held transmitter/receiver probe. The device creates a mild (1 cm) artifact on MRI. The aim of this systematic review is to synthesise published information on MRI burns and safety-related guidance to develop comprehensive safety recommendations for best MRI practice to prevent burns. It concludes: “According to the ASTM standard, a device is considered as MR-safe if it causes no known hazards to patients in all MR environments. Since the RFID tag contains conducting materials, RFID may only be MR-conditional, meaning safe under certain conditions for MR imaging during the scan. Significant increasing of temperature and . MRI safety. radiofrequency identification. RFID. R adio-frequency identification (RFID) technology has been in use for over 50 years. The technology involves a microchip attached to an antenna, which responds to an incoming signal from a reader by sending an outgoing signal.
Although some implant devices with RFID tags may safely undergo MRI on the basis of formal safety testing, peer-reviewed literature on RFID chip safety and impact on image quality is scant, and MRI should be performed with caution [2].
The radio frequency identification (RFID) technology with tags (transponders) in wristbands and medical equipment may close this gap and increase patient safety. An RFID tag is a microchip attached to an antenna and usually fashioned in such a . A recent development has been the labeling of the implants with a radio-frequency device micro responder chip (RFID). We examined a patient with silicone implants containing RFID chips with magnetic resonance imaging and were surprised by .
Patients wearing RFID wristbands are safe in 1.5 T and 3 T MR scanners using normal operation mode for RF-field. The findings are specific to the RFID tags that underwent testing.
Android-powered devices are usually looking for NFC tags when the screen is unlocked, unless NFC is disabled in the device's Settings menu. When an Android-powered device discovers an NFC tag, the desired behavior .
rfid chip mri safety|verichip microtransponder mri