fda approved rfid tags Because this technology continues to evolve and is more widely used, it is important to keep in mind its potential for interference with pacemakers, implantable cardioverter defibrillators (ICDs), and other electronic medical devices. Physicians should stay informed . See more $24.98
0 · usda free rfid tags
1 · rfid tags livestock
2 · official USDA cattle id tags
3 · goat ear tags scrapie usda
4 · federal 840 identification tags
5 · electronic identification tags for cattle
6 · do goats need scrapie tags
7 · 840 livestock tags
$11.99
Radio Frequency Identification (RFID) refers to a wireless system comprised of two components: tags and readers. The reader is a device that has one or more antennas that emit radio waves and receive signals back from the RFID tag. Tags, which use radio waves to communicate their identity and other information . See more
RFID systems use radio waves at several different frequencies to transfer data. In health care and hospital settings, RFID technologies include the following applications: 1. . See moreThe FDA has taken steps to study RFID and its potential effects on medical devices including: 1. Working with manufacturers of potentially susceptible medical devices to test their products for any adverse effects from RFID and encouraging them to consider RFID . See more
Because this technology continues to evolve and is more widely used, it is important to keep in mind its potential for interference with pacemakers, implantable cardioverter defibrillators (ICDs), and other electronic medical devices. Physicians should stay informed . See morePrompt reporting of adverse events can help the FDA identify and better understand the risks associated with RFID. If you suspect a problem, we encourage you to file . See more
A manufacturer, repackager, relabeler, distributor, retailer, or others acting at their direction will attach RFID tags (chips and antennae) to only immediate containers, secondary packaging,. The U.S. Food and Drug Administration has approved the country's first radio-frequency identification chip that can be implanted in humans. The 134.2-KHz RFID chips .The US Food and Drug Administration has approved Verichip, an implantable radiofrequency identification device for patients, which would enable doctors to access their medical records.Radio Frequency Identification (RFID) refers to a wireless system comprised of two components: tags and readers. The reader is a device that has one or more antennas that emit radio waves and.
A manufacturer, repackager, relabeler, distributor, retailer, or others acting at their direction will attach RFID tags (chips and antennae) to only immediate containers, secondary packaging,.
The tags allow the test kits to be authenticated as Food and Drug Administration (FDA)-approved and from a legitimate source. The HIPAA-compliant platform also provides test results, which clinicians with permission can access for greater visibility. The U.S. Food and Drug Administration has approved the country's first radio-frequency identification chip that can be implanted in humans. The 134.2-KHz RFID chips could save lives and possibly limit injuries from errors in medical treatments, according to VeriChip Corp., a subsidiary of Applied Digital Solutions Inc.The US Food and Drug Administration has approved Verichip, an implantable radiofrequency identification device for patients, which would enable doctors to access their medical records.
Exceed industry standards with invisible, permanent tags lasting as long as a device. Mitigate degradation from rough handling, abrasion, cleaning, sterilization or other hazards. Marry UID of RFID tag to FDA UDI number for streamlined traceability.
Fresenius Kabi +RFID™ medicines will feature a high-performance RFID tag embedded in the label that contains the relevant data that hospitals rely on every day to immediately identify, locate and manage their inventory.Keren Sookne. Mar 8, 2021. With the CCL/Kit Check partnership, manufacturers receive labels with RFID inlay that appear identical to their existing label—and don’t require artwork changes and new FDA approval—and do not affect packaging lines. The new EOS-202 U9 high-performance UHF product underlines Tageos’ commitment to deliver innovative, high-quality RFID inlays, confirmed by the company’s award of the ARC Quality Certification for the design and manufacture of its RFID inlays and tags.The U.S Food and Drug Administration (FDA) has recently approved a tag called VeriChip for use in humans. These tiny tags help disoriented, elderly and high risk patients more secure status by storing a full medical record.
Radio Frequency Identification (RFID) refers to a wireless system comprised of two components: tags and readers. The reader is a device that has one or more antennas that emit radio waves and.A manufacturer, repackager, relabeler, distributor, retailer, or others acting at their direction will attach RFID tags (chips and antennae) to only immediate containers, secondary packaging,. The tags allow the test kits to be authenticated as Food and Drug Administration (FDA)-approved and from a legitimate source. The HIPAA-compliant platform also provides test results, which clinicians with permission can access for greater visibility.
rfid card dispenser
The U.S. Food and Drug Administration has approved the country's first radio-frequency identification chip that can be implanted in humans. The 134.2-KHz RFID chips could save lives and possibly limit injuries from errors in medical treatments, according to VeriChip Corp., a subsidiary of Applied Digital Solutions Inc.The US Food and Drug Administration has approved Verichip, an implantable radiofrequency identification device for patients, which would enable doctors to access their medical records.Exceed industry standards with invisible, permanent tags lasting as long as a device. Mitigate degradation from rough handling, abrasion, cleaning, sterilization or other hazards. Marry UID of RFID tag to FDA UDI number for streamlined traceability. Fresenius Kabi +RFID™ medicines will feature a high-performance RFID tag embedded in the label that contains the relevant data that hospitals rely on every day to immediately identify, locate and manage their inventory.
Keren Sookne. Mar 8, 2021. With the CCL/Kit Check partnership, manufacturers receive labels with RFID inlay that appear identical to their existing label—and don’t require artwork changes and new FDA approval—and do not affect packaging lines. The new EOS-202 U9 high-performance UHF product underlines Tageos’ commitment to deliver innovative, high-quality RFID inlays, confirmed by the company’s award of the ARC Quality Certification for the design and manufacture of its RFID inlays and tags.
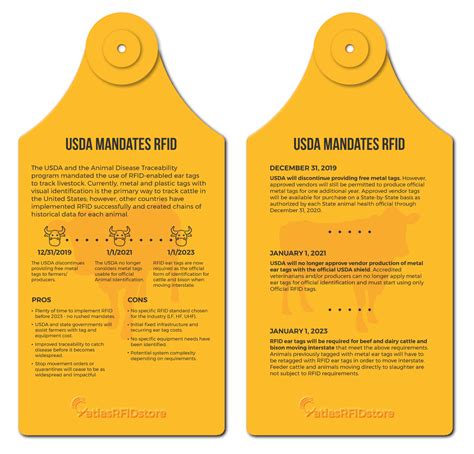
usda free rfid tags

Digital smart card with 3.7 inch e-ink display , mobile APP to update by NFC. .
fda approved rfid tags|electronic identification tags for cattle